**Cellulose History and Structure:**
– Discovered in 1838 by Anselme Payen.
– Used for celluloid production in 1870.
– Rayon production began in the 1890s.
– Cellophane invented in 1912.
– Polymer structure determined by Hermann Staudinger in 1920.
– Insoluble in water and most organic solvents.
– Melts at 467°C.
– Forms hydrogen bonds for high tensile strength.
– Various cellulose structures known (I, II, III, IV).
– Chain lengths range from 300 to 10,000 units.
**Cellulose Processing and Biosynthesis:**
– Synthesized at the plasma membrane by RTCs.
– RTCs contain cellulose synthase enzymes.
– CesA genes involved in biosynthesis.
– Seven subfamilies in the plant CesA superfamily.
– Cellulose nanofibrils and nanocrystals produced.
– Cellulose synthesized by RTCs at the plasma membrane.
– RTCs are hexameric protein structures.
– CesA genes involved in primary and secondary biosynthesis.
– Cellulose synthases use UDP-glucose for synthesis.
– Cellulose synthases belong to glucosyltransferase family 2 (GT2).
**Cellulose Applications and Uses:**
– Electrical insulation paper for transformers and cables.
– Main ingredient in textiles like cotton.
– Used in drug tablets and processed foods.
– Building materials like insulation and sprayable material.
– Converted into cellophane and water-soluble adhesives.
– Used in highly absorbent sponges and nitrocellulose production.
– Historical use in photographic and movie films.
– Beneficial in pharmaceutical formulations and energy crops.
**Cellulose in Technology and Environmental Impact:**
– Nanocrystalline cellulose and biobased nanocomposites.
– Fungal cellulases in various industries.
– Sustainable electronics and eco-friendly devices.
– Cellulose breakdown by gut bacteria and termites.
– Pyrolysis challenges for biofuels and plant materials study.
– Ionic liquid processing, cellulose ethers, and engineered surfaces.
– Ruminococci and horse digestive functions related to cellulose.
**Advanced Cellulose Applications:**
– Sustainable hydroxypropyl cellulose-nanodiamond composites.
– Thiolated cellulose polymers for drug delivery.
– Cellulose in industrial applications like insulation and biofuels.
– Environmental sustainability through cellulose-based solutions.
– Cellulose resources, references, and applications in robotics.
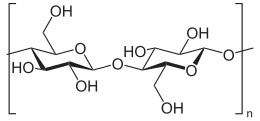
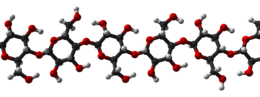
Cellulose is an organic compound with the formula (C
6H
10O
5)
n, a polysaccharide consisting of a linear chain of several hundred to many thousands of β(1→4) linked D-glucose units. Cellulose is an important structural component of the primary cell wall of green plants, many forms of algae and the oomycetes. Some species of bacteria secrete it to form biofilms. Cellulose is the most abundant organic polymer on Earth. The cellulose content of cotton fiber is 90%, that of wood is 40–50%, and that of dried hemp is approximately 57%.
| |
| |
| Identifiers | |
|---|---|
| ChEMBL | |
| ChemSpider |
|
| ECHA InfoCard | 100.029.692 |
| EC Number |
|
| E number | E460 (thickeners, ...) |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| Properties | |
| (C 12H 20O 10) n | |
| Molar mass | 162.1406 g/mol per glucose unit |
| Appearance | white powder |
| Density | 1.5 g/cm3 |
| Melting point | 260–270 °C; 500–518 °F; 533–543 K Decomposes |
| none | |
| Thermochemistry | |
Std enthalpy of
formation (ΔfH⦵298) |
−963,000 kJ/mol[clarification needed] |
Std enthalpy of
combustion (ΔcH⦵298) |
−2828,000 kJ/mol[clarification needed] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
TWA 15 mg/m3 (total) TWA 5 mg/m3 (resp) |
REL (Recommended)
|
TWA 10 mg/m3 (total) TWA 5 mg/m3 (resp) |
IDLH (Immediate danger)
|
N.D. |
| Related compounds | |
Related compounds
|
Starch |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Cellulose is mainly used to produce paperboard and paper. Smaller quantities are converted into a wide variety of derivative products such as cellophane and rayon. Conversion of cellulose from energy crops into biofuels such as cellulosic ethanol is under development as a renewable fuel source. Cellulose for industrial use is mainly obtained from wood pulp and cotton. Cellulose is also greatly affected by direct interaction with several organic liquids.
Some animals, particularly ruminants and termites, can digest cellulose with the help of symbiotic micro-organisms that live in their guts, such as Trichonympha. In human nutrition, cellulose is a non-digestible constituent of insoluble dietary fiber, acting as a hydrophilic bulking agent for feces and potentially aiding in defecation.

