**Chemical Properties of Ammonium:**
– Ammonium ion is generated from ammonia in the presence of Brønsted acids.
– It is mildly acidic and can react with bases to form uncharged ammonia.
– Equilibrium between ammonia and ammonium ions depends on solution pH.
– Treatment of ammonium salts with a strong base yields ammonia.
– Ammonium cation is found in salts like ammonium carbonate, chloride, and nitrate.
– Ammonium salts exhibit characteristic reactions, such as releasing ammonia gas when heated with alkali hydroxide.
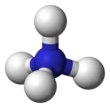
– The lone electron pair on nitrogen in ammonia forms a coordinate bond with a proton.
– The size and structure of the ammonium cation are similar to the caesium cation.
– Ammonium cation has polar covalent bonds.
**Organic Ammonium Compounds:**
– Ammonium ions can be substituted with organic groups to form various types of ammonium ions.
– Different organic groups determine the classification of ammonium cations as primary, secondary, tertiary, or quaternary.
– Quaternary ammonium cations are devoid of a hydrogen atom and are used to enhance solubility in organic solvents.
– Various types of organic ammonium salts serve as phase-transfer catalysts and surfactants.
– Organic ammonium salts include derivatives of amine radical cations.
**Biological Role and Environmental Impact of Ammonium:**
– Ammonium ions are waste products in animal metabolism.
– Plants utilize ammonium as a nitrogen source, but it can be toxic to most crop species.
– Fish excrete ammonium directly into water, while mammals convert it to urea.
– Birds and reptiles convert metabolic ammonium into uric acid.
– Ammonium runoff from fertilizers can contribute to water pollution.
**Industrial Applications and Preparation of Ammonium:**
– Ammonium is synthesized by reacting ammonia with acids.
– The Haber process is a common industrial method for ammonium production.
– Ammonium is essential in agriculture as a nutrient for plants.
– It is used in fertilizers, cleaning products, chemical manufacturing, pharmaceuticals, textiles, and the food industry.
– Laboratory preparation involves mixing ammonia with a strong acid to form ammonium salts.
**Safety and Reactions Involving Ammonium:**
– Ammonium compounds can be toxic if ingested and corrosive to the skin.
– Inhalation of ammonia gas from ammonium compounds can irritate the respiratory system.
– Storage precautions include keeping ammonium away from oxidizing agents.
– Ammonium reacts in various ways, including forming salts in acid-base reactions, releasing ammonia gas upon heating, burning in the presence of oxygen, and complexing with transition metals.
The ammonium cation is a positively charged polyatomic ion with the chemical formula NH+4 or [NH4]+. It is formed by the protonation of ammonia (NH3). Ammonium is also a general name for positively charged (protonated) substituted amines and quaternary ammonium cations ([NR4]+), where one or more hydrogen atoms are replaced by organic or other groups (indicated by R).
| |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Ammonium ion
| |||
| Systematic IUPAC name
Azanium | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChemSpider | |||
| MeSH | D000644 | ||
PubChem CID
|
|||
| UNII | |||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| [NH4]+ | |||
| Molar mass | 18.039 g·mol−1 | ||
| Acidity (pKa) | 9.25 | ||
| Conjugate base | Ammonia | ||
| Structure | |||
| Tetrahedral | |||
| Related compounds | |||
Other cations
|
| ||
Related compounds
|
Ammonium radical •NH4 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||