**Structure and Classification of Tannins:**
– Base units/scaffolds: Gallic acid, Phloroglucinol, Flavan-3-ol
– Polymer classes: Hydrolyzable tannins, Phlorotannins, Condensed tannins, Phlobatannins
– Sources: Plants, Brown algae, Tree heartwood
– Oligostilbenoids as oligomeric forms of stilbenoids
**History and Occurrence of Tannins:**
– Discovery by Henri Braconnot in 1831 (ellagic acid, gallic acid, pyrogallic acid)
– Julius Löwe’s synthesis of ellagic acid
– Maximilian Nierenstein’s studies on natural phenols and tannins
– Presence in gymnosperms and angiosperms, various plant families
– Abundance of condensed tannins, specific plant families like Aceraceae, Actinidiaceae
**Cellular Localization and Extraction of Tannins:**
– Tannin production in tannosomes, storage in vacuoles or surface wax
– Classification as ergastic substances, presence in idioblasts
– Leaching of water-soluble tannins, effects on water and wood
– Extraction methods and variations, use of acetone for enhanced yield
**Analytical Methods and Health Benefits of Tannins:**
– Tests for tannins: precipitation, reaction with phenolic rings, depolymerization
– Alkaloid precipitation for quantitation, Goldbeaters skin test, Ferric chloride test
– Hide-powder and Stiasny methods, other colorimetric methods for quantification
– Antimicrobial, antioxidant properties, nutritional toxicology, health implications
**Uses and Applications of Tannins:**
– Historical and industrial uses in tanning, leather preservation, and iron artifacts
– Market trends, decline in vegetable tannins, uses in beverages and clarifying agents
– Animal nutrition properties, impact on silage quality, fermentability, and digestion
– Culinary and beverage uses, industrial applications in adhesives, anti-corrosives
– Medical and environmental uses, research publications, and external links related to tannins
Tannins (or tannoids) are a class of astringent, polyphenolic biomolecules that bind to and precipitate proteins and various other organic compounds including amino acids and alkaloids.
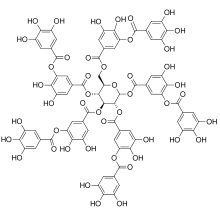


The term tannin (from Anglo-Norman tanner, from Medieval Latin tannāre, from tannum, oak bark) refers to the use of oak and other bark in tanning animal hides into leather. By extension, the term tannin is widely applied to any large polyphenolic compound containing sufficient hydroxyls and other suitable groups (such as carboxyls) to form strong complexes with various macromolecules.
The tannin compounds are widely distributed in many species of plants, where they play a role in protection from predation (acting as pesticides) and might help in regulating plant growth. The astringency from the tannins is what causes the dry and puckery feeling in the mouth following the consumption of unripened fruit, red wine or tea. Likewise, the destruction or modification of tannins with time plays an important role when determining harvesting times.
Tannins have molecular weights ranging from 500 to over 3,000 (gallic acid esters) and up to 20,000 daltons (proanthocyanidins).
